electron configuration for iron|Electron Configuration for Iron (Fe, Fe2+, and Fe3+) : Manila Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s . Curious_village_title.jpg In this section, you will find detailed information regarding every puzzle in the game including locations, picarats, hints, as well as solutions. The entries are once .What is the Sky Bet free bets promotion? Like most bookmakers and online casinos, Sky Bet has a range of promotions that are available for customers to take advantage of. Most are only available to those just signing up to use the company’s services for the first time, but existing customers may also be able to take advantage of some other promotions.
PH0 · What is the electron configuration of iron?
PH1 · Iron Electron Configuration (Fe) with Orbital Diagram
PH2 · Iron
PH3 · Electronic Configuration of Iron: Fe element
PH4 · Electronic Configuration of Iron
PH5 · Electron configuration for Iron (element 26). Orbital diagram
PH6 · Electron Configuration for Iron (Fe, Fe2+, and Fe3+)
PH7 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH8 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH9 · Electron Configuration Chart of All Elements (Full Chart)
Isinasagawa na ang pilot test ng printing ng electronic version ng national ID na magiging alternatibo habang wala pa ang physical ID.. Ito ang inihayag ni Dennis Mapa, ang n ational statistician and civil registar general ng Philippine Statistics Authority, noong October 3, 2022.. Ani Mapa, target ng Philippine Statistics Authority na makapag .
electron configuration for iron*******Learn how to write the electron configuration for iron and its ions using the period table or an electron configuration chart. See the video, examples and step-by-step tutorial for writing the electron configurations.In order to write the Calcium electron configuration we first need to know the .Electron Configuration for Iron (Fe, Fe2+, and Fe3+) Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .
How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .
When we write the configuration we'll put all 19 electrons in orbitals around the . Mar 23, 2023
Electronic Configuration of Iron. The chemical element iron has the atomic number 26 and the symbol Fe (from Latin: Ferrum). It’s a transition metal from .
Learn how to write the electronic configuration of iron and its oxidation states, valency, and applications. See examples, diagrams, and FAQs on iron's atomic structure and properties. Learn how to write the electron configuration of iron using the noble gas shortcut and the energy levels of the orbitals. See the answer, the long version, and the corrected diagram. In order to write the Iron Electron Configuration, we need to know the number of electrons for the GFe atom, and once we have the configuration for Fe, the ions are simple. .Electronic configuration of the Iron atom. Valence electrons. Orbital diagram. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, the Iron electron configuration is basically the 1s2 2s2 2p6 3s2 .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. Boiling pointThe .Aufbau Principle. Aufbau comes from the German "structure" or building up and Aufbauprinzip, is the "building up principle" where we assign electrons to the lowest energy orbitals first, and then successively add them to higher energy .The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron .
The ground state electron configuration of Fe is: "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^6"4s"^2" For all but about 20 transition metals, the Aufbau diagram is a useful tool that helps to determine the ground .electron configuration for ironElement Iron (Fe), Group 8, Atomic Number 26, d-block, Mass 55.845. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element .electron configuration for iron Electron Configuration for Iron (Fe, Fe2+, and Fe3+) The electron configurations for Cations are also made based on the number of electrons but there is a slight difference in the way they are configured. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. . Iron has 26 electrons so its normal electron .The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. . Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital Fe 2+: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 = 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6.

In the case of Fe, which represents the element iron, the orbital diagram illustrates the electron configuration of the iron atom. The electron configuration of Fe can be represented using the noble gas shorthand method. Iron has an atomic number of 26, which means it has 26 electrons. The noble gas shorthand for Fe can be written as [Ar] 4s2 3d6.For example, the electron configuration of iron is Fe is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated electron configuration (or noble gas configuration) to identify valence .
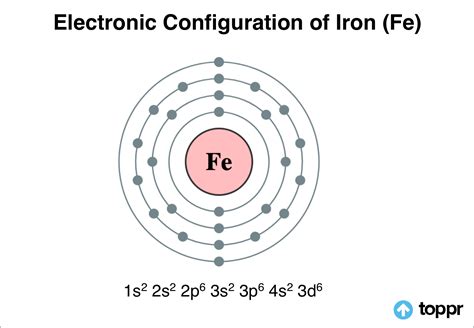
Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure.The chemical symbol for Iron is Fe. Electron Configuration and Oxidation States of Iron. Electron configuration of Iron is [Ar] 3d6 4s2. Possible oxidation states are +2,3. Electron Configuration. The periodic table is a tabular .Figure \(\PageIndex{7}\): A core-abbreviated electron configuration (right) replaces the core electrons with the noble gas symbol whose configuration matches the core electron configuration of the other element. . Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital Fe 2+: .The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels, this added stability is enough to shift an electron from one orbital to another. In heavier elements .Electronic configuration of the Iron atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2 Reduced electronic configuration Fe: [Ar] 3d 6 4s 2. Below is the electronic diagram of the Iron atom Distribution of electrons over energy levels in the Fe atom 1-st level (K): 2If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.Its 26 electrons are arranged in the configuration [Ar]3d 6 4s 2, of which the 3d and 4s electrons are relatively close in energy, and thus a number of electrons can be ionized. [16] Iron forms compounds mainly in the oxidation states +2 (iron(II), "ferrous") and +3 (iron(III), "ferric").
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 . To write the orbital diagram for the Iron (Fe) first we need to write the electron configuration for just Fe. To do that we need to find the number of elect.
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and 2p subshells . The iron orbital diagram is a graphical representation of the electron configuration of the iron atom.. This diagram shows how the electrons in the iron atom are arranged in different orbitals.
Registering in SSS as employer or business owner have slightly different steps depending on the company formation. Single Proprietorships. An owner of a single proprietorship business should accomplish and submit SSS Form R-1 (Employer SSS Registration Firm) and R-1A (SSS Employment Report).What is the puck line in sports betting? This guide explains what puck line means, how it works and how you can place a puck line wager. What is puck line betting? Puck line betting is a method of betting on hockey. It is one of the most popular markets for hockey betting. Puck line betting is more commonly known as spread .
electron configuration for iron|Electron Configuration for Iron (Fe, Fe2+, and Fe3+)